

Cell culture contamination control: Detection
Detection and identification of Mycoplasma
Venor®GeM-qEP Mycoplasma Detection Kit
Venor®GeM qEP utilizes quantitative, real-time PCR (qPCR). The kit can be performed with any type of real-time PCR cycler able to detect the fluorescence dyes FAM™ and HEX™. The protocol provided is preferred for fast and reliable screening of cell culture supernatants most applicable in research and development. The detection procedure can be performed within 3 hours.Important note: Please see the note section for important information on the use of the supplied positive control
Minerva Biolabs
| Catalogue No. | Description | Pack Size | Price | Qty |
|
|---|---|---|---|---|---|
| 11-9025 | Venor®GeM qEP | 25 rxns | £283.00 | Quantity | Add to Order |
| 11-9100 | Venor®GeM qEP | 100 rxns | £933.00 | Quantity | Add to Order |
| 11-9250 | Venor®GeM qEP | 250 rxns | £1,894.00 | Quantity | Add to Order |
| 11-9905 | Internal Control DNA extra for VenorGem qEP | 4 vials | £19.00 | Quantity | Add to Order |
Venor®GeM-qEP Mycoplasma Detection Kit
Venor®GeM qEP utilizes quantitative, real-time PCR (qPCR). The kit can be performed with any type of real-time PCR cycler able to detect the fluorescence dyes FAM™ and HEX™. The protocol provided is preferred for fast and reliable screening of cell culture supernatants most applicable in research and development. The detection procedure can be performed within 3 hours.Important note: Please see the note section for important information on the use of the supplied positive control
Minerva Biolabs
Venor®GeM qEP
Mycoplasmas are specifically detected by amplifying a highly conserved rRNA operon, or more specifically, the 16S rRNA coding region in the mycoplasma genome. The mycoplasma-specific amplification is detected at 520 nm (FAM™ channel).The kit includes primer and FAM™ labeled probes which allow the specific detection of all Mollicutes species so far described as contaminants of cell cultures and media components. Eukaryotic DNA is not amplified by this primer/probe system. False negative results due to PCR inhibitors or improper DNA extraction are detected by the internal amplification control which can be added to the PCR master mix. The amplification of the internal amplification control is detected at 610 nm (HEX™ channel).
Important note:
Please see the note section for important information on the use of the supplied positive control
Type of PCR
TaqMan® basedqPCR Assay with FAM™ and HEX™ labeled probes
Recommended Use / Scope
Venor®GeM qEP is used for direct detection of Mollicutes (Mycoplasma, Acholeplasma, Spiroplasma) contamination in cell cultures and cell media components.
Kit Components
lyophilized primer/nucleotide/probe/polymerase/mix in aliquots of 25 tests rehydration bufferlyophilized internal amplification control lyophilized positive control PCR grade water
Package Sizes
- Cat. No. 11-9025 25 tests
- Cat. No. 11-9100 100 tests
- Cat. No. 11-9250 250 tests
Result Evaluation
cycler based, real-time PCR
Required Consumables
PCR reaction tubes Optional for process validation and EP 2.6.7 compliant testing: Internal Control DNA extra (4 vials for 300 µl each of internal amplification control; Cat. No. 11-9905) 10CFU™ Sensitivity Standards available for all EP2.6.7 listed mycoplasma species
Required Lab Devices
qPCR cycler with FAM™ and HEX™ filter variable microliter pipettes benchtop centrifuge for 1.5 ml reaction tubes
Shelf Life and Storage
Components can be stored at2 to8 °C for at least 12 months. After rehydration the reagents must be stored at -18 °C.
EP 2.6.7 Compliance
Yes.Please note that validation data are provided for information purpose only. EP 2.6.7 clearly states “Where commercial kits are used …, documented validation points already covered by the kit manufacturer can replace validation by the user. Nevertheless, the performance of the kit with respect to its intended use has to be demonstrated by the user (e.g. detection limit, robustness, cross-detection of other classes of bacteria.”. Please feel free to contact us if you need further assistance.
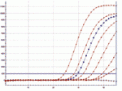 Fig. Amplified dilution series of Mycoplasma fermentans, performed on a Corbett RotorGene®6000.
Fig. Amplified dilution series of Mycoplasma fermentans, performed on a Corbett RotorGene®6000.
If you cannot find the answer to your problem below then please contact us or telephone 01954 210 200
Venor®GeM-qEP Mycoplasma Detection Kit
Venor®GeM qEP utilizes quantitative, real-time PCR (qPCR). The kit can be performed with any type of real-time PCR cycler able to detect the fluorescence dyes FAM™ and HEX™. The protocol provided is preferred for fast and reliable screening of cell culture supernatants most applicable in research and development. The detection procedure can be performed within 3 hours.Important note: Please see the note section for important information on the use of the supplied positive control
Minerva Biolabs
Venor®GeM qEP protocol
Quick Visual Protocol
Please note: all protocols off site are the responsibility of the products supplier
If you cannot find the answer to your problem below then please contact us or telephone 01954 210 200
Venor®GeM-qEP Mycoplasma Detection Kit
Venor®GeM qEP utilizes quantitative, real-time PCR (qPCR). The kit can be performed with any type of real-time PCR cycler able to detect the fluorescence dyes FAM™ and HEX™. The protocol provided is preferred for fast and reliable screening of cell culture supernatants most applicable in research and development. The detection procedure can be performed within 3 hours.Important note: Please see the note section for important information on the use of the supplied positive control
Minerva Biolabs
For a list of citations please click here
If you cannot find the answer to your problem below then please contact us or telephone 01954 210 200
Venor®GeM-qEP Mycoplasma Detection Kit
Venor®GeM qEP utilizes quantitative, real-time PCR (qPCR). The kit can be performed with any type of real-time PCR cycler able to detect the fluorescence dyes FAM™ and HEX™. The protocol provided is preferred for fast and reliable screening of cell culture supernatants most applicable in research and development. The detection procedure can be performed within 3 hours.Important note: Please see the note section for important information on the use of the supplied positive control
Minerva Biolabs
Important notes on the use postive controls in the VenorGem™ QEP kit
The Positive Control comes in one vial. After rehydration, this reagent is stable for frequent thawing/freezing cycles. However we strongly recommend that customers use DNase-free pipette tips and a clean surrounding. One bacteria particle contaminating the reagent is able to digest all the DNA during the thawing phase.
Aliquoting the rehydrated Positive Control can help avoid this as long as the following criterion are met: 1. Make aliquots in PCR tubes (they are usually clean, DNase-free, low-binding tubes, low surface area). 2. Never ever use reaction tubes or other larger vials, as they absorb the minute amounts of DNA 3. keep the thawing time short. Freeze asap.
Required consumables:
PCR reaction tubes
Optional for process validation and EP 2.6.7 compliant testing:
Internal Control DNA extra (4 vials for 300 µl each of internal amplification control; Cat. No. 11-9905)
10CFU™ Sensitivity Standards available for all EP listed mycoplasma species
Required lab devices:
qPCR cycler with FAM™ and ROX™ filter
variable microliter pipettes
benchtop centrifuge for 1.5 ml reaction tubes
Shelf life and storage:
Components can be stored at2 to8 °C for at least 12 months. After rehydratisation the reagents must be stored at -18 °C.
EP 2.6.7 Compliance:
Yes.
Please note that validation data are provided for information purpose only. EP 2.6.7 clearly states “Where commercial kits are used …, documented validation points already covered by the kit manufacturer can replace validation by the user. Nevertheless, the performance of the kit with respect to its intended use has to be demonstrated by the user (e.g. detection limit, robustness, cross-detection of other classes of bacteria.”. Please feel free to contact us if you need further assistance.
If you cannot find the answer to your problem below then please contact us or telephone 01954 210 200
Venor®GeM-qEP Mycoplasma Detection Kit
Venor®GeM qEP utilizes quantitative, real-time PCR (qPCR). The kit can be performed with any type of real-time PCR cycler able to detect the fluorescence dyes FAM™ and HEX™. The protocol provided is preferred for fast and reliable screening of cell culture supernatants most applicable in research and development. The detection procedure can be performed within 3 hours.Important note: Please see the note section for important information on the use of the supplied positive control
Minerva Biolabs
If you cannot find the answer to your problem below then please contact us or telephone 01954 210 200



